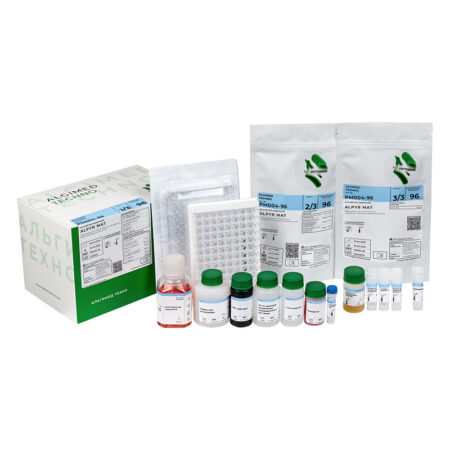
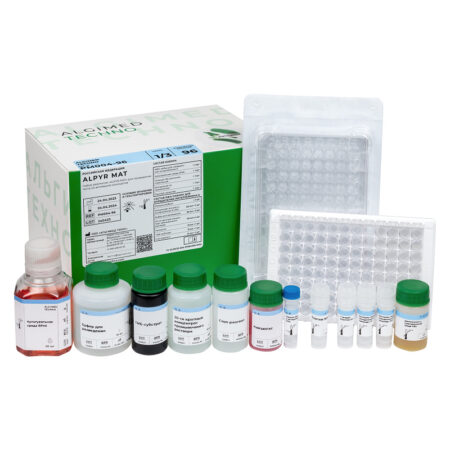


«ALPYR MAT» monocyte activation test kit
Pharmaceutical products may contain pyrogens, the sources of which may be bacteria, viruses, fungi, and pyrogenes may also be by-products of the production process. The pharmaceutical product contamination with pyrogens is a serious threat to the health of patients and can lead to fever and, as a result, to shock and death. Currently, the pyrogenicity analysis for the pyrogenic substance content determination (bacterial endotoxins and non-endotoxin substances) is carried out on rabbits. It is inaccurate and harmful to animals. The Monocyte Activation Test (MAT) has a higher sensitivity and does not require the use of animals.
PDF for download
360° Photo
| Name | Features | Quantity |
| MAT cells |
Cryopreserved monocytes obtained from peripheral blood from four different donors. The use of cryopreserved monocytes eliminates the need to work with donated blood in the laboratory. One bottle is enough for one 96-well plate analysis. Cells are stored at -80 ° C and below for 6 months. |
1 vial x 1 ml |
| RPMI culture medium I |
Designed for monocytes dilution, as well as for preparing dilutions of test products, endotoxin standards and non-endotoxin controls. |
1 bottle x 50 ml |
| Supplement to FBS culture medium |
Designed for the culture medium preparation for the cryopreserved monocytes dilution after thawing. |
1 bottle x 5 ml |
| Endotoxin Standard |
International Endotoxin Standard RSE. Designed for building a calibration curve and setting a positive control of the product. |
1 vial x 50 μl |
| HKSA Non-endotoxin pyrogen control | Thermoinactivated Staphylococcus aureus. Designed for preparation of non-endotoxin pyrogen controls. | 1 vial x 50 μl |
| LTA Non-endotoxin pyrogen control | Lipoteichoic acid. Designed for preparation of non-endotoxin pyrogen controls. | 1 vial x 50 μl |
| Interleukin-6 ELISA Kit | A ready-made ELISA kit for the determination of interleukin-6. The kit includes one 96-well plate for ELISA, containing antibodies to interleukin-6 and the necessary reagents. The calibration curve is plotted against the endotoxin standard. Interleukin-6 standard must be ordered separately if required. | 1 pcs |
| 96-well plate, sterile | Sterile pyrogen-free flat-bottomed 96-well plate with lid. Designed for incubation of tested products and endotoxin standards with monocytes. | 1 pcs |
The Monocyte Activation Test (MAT) has been developed and validated as a method for identifying and quantifying bacterial endotoxins and pyrogens of unknown origin that activate human monocytes, resulting in the release of endogenous mediators. As a source of monocytes, the reagent kit includes cryopreserved mononuclear cells of human peripheral blood, which makes it possible to determine the minimum amount of pyrogenic substances. The test is carried out by incubating freshly thawed monocytes with the test product solutions for a day, and then the released interleukin-6 is measured, which serves as an indicator of monocyte activation caused by pyrogens. The standard for bacterial endotoxin is lipopolysaccharide (LPS), obtained from gram-negative bacteria, and its content is expressed in international endotoxin units. Lipoteichoic acid and heat-inactivated Staphylococcus aureus are used as non-endotoxin controls.