

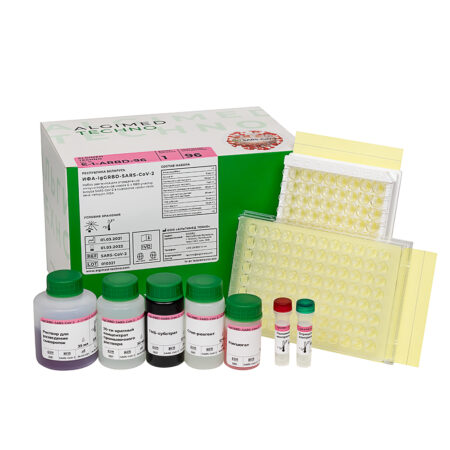

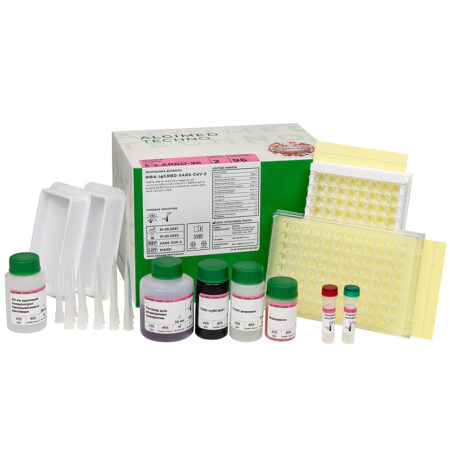
«IgG RBD-SARS-CoV-2 ELISA» reagent kit
«IgG RBD-SARS-CoV‑2 ELISA» reagent kit for the qualitative detection of IgG to the SARS-CoV-2 glycoprotein S (spike) receptor-binding domain (RBD): IgGRBD-SARS-CoV‑2 ELISA Kit. «IgG RBD-SARS-CoV‑2 ELISA» reagent kit manufactured by Algimed Techno is intended for in vitro use only.
PDF for download
Specific IgG to the SARS-CoV-2 glycoprotein S RBD have virus neutralizing activity by blocking the binding of the virus to the ACE2 human cell receptor. Information about the content of such immunoglobulins in the patient blood serum or plasma leads to the following conclusions:
- Reinfection risk assessment.
- Donor plasma suitability for transfusion in patients with severe coronavirus infection.
- Vaccination effectiveness.
How to determine antibodies to the SARS-CoV-2 coronavirus
360° Photo
The principle is based on indirect noncompetitive two-step ELISA. At first step samples are incubated in the microtiter plate, precoated with a recombinant SARS-CoV‑2 RBD. Immunoglobulins to this protein when present in the sample bind specifically to the antigen immobilized in the wells. Next, IgG-class antibodies are detected with enzyme conjugate (HRP-labelled anti-human IgG antibody). After incubation with the TMB Substrate and stop solution application, the solution optical density is measured. The measured optical density is directly related to the number of IgG to SARS-CoV‑2 RBD in the samples.
- E-1-ARBD-96
- E-2-ARBD-96
«IgG RBD-SARS-CoV-2 ELISA» reagent kit (c.n. E-1-ARBD-96, for 96 determinations):
- Microtiter plate – 1 pc;
- Conjugate – 11 mL × 1 pc;
- Positive Control – 0,5 mL × 1 pc;
- Negative Control – 1 mL × 1 pc;
- Sample Diluent – 35 mL × 1 pc;
- Wash Concentrate (20x)* – 30 mL × 1 pc;
- TMB Substrate* – 12 mL × 1 pc;
- Stop Solution* – 12 mL × 1 pc;
- Dilution plate – 1 pc;
- Plate coat – 2 pcs;
«IgG RBD-SARS-CoV-2 ELISA» reagent kit (c.n. E-2-ARBD-96, for 96 determinations):
- Microtiter plate – 1 pc;
- Conjugate – 11 mL × 1 pc;
- Positive Control – 0,5 mL × 1 pc;
- Negative Control – 1 mL × 1 pc;
- Sample Diluent – 35 mL × 1 pc;
- Wash Concentrate (20x)* – 30 mL × 1 pc;
- TMB Substrate* – 12 mL × 1 pc;
- Stop Solution* – 12 mL × 1 pc;
- Dilution plate – 1 pc;
- Plate coat – 2 pcs;
- Tray for multichannel pipettors – 2 pcs;
- Disposable polymer pipettе tip – 16 pcs;
* The following kit components are interchangeable within their storage life, provided that their series number coincides in the kits produced by Algimed Techno LLC.
Verification of positive results for antibody to SARS-CoV-2
Temporary guidelines (Version 8 dated September 3, 2020) prescribe the use of test systems that determine antibody (IgG) to RBD.
Determination of protective antibody against SARS-CoV-2 in those who had contracted coronavirus infection
To assess the reinfection risk and to determine if a patient needs to be vaccinated.
Determination of protective antibody in vaccinated
Antibody formation control in the post vaccination period.