

«ELISA AG SARS-CoV-2» reagent kit
«ELISA AG SARS-CoV‑2» reagent kit is intended for the detection of the SARS CoV‑2 antigen (nucleocapsid protein) in clinical samples (nasopharyngeal swab, oropharyngeal swab) for the diagnosis of coronavirus disease (COVID-19), differential diagnosis of the acute respiratory infection etiology. The kit is intended for in vitro use only.
PDF for download
The specific SARS-CoV‑2 nucleocapsid protein makes up the largest proportion of the virus’s structural proteins of the and is the most abundant protein in infected cells. Based on the information on the content of this antigen in the clinical samples of patients it is possible to draw conclusions about:
- The presence of coronavirus infection (COVID-19).
- Effectiveness of coronavirus infection (COVID-19) therapy.
360° Photo
Advantages of «ELISA AG SARS-CoV-2» reagent kit in detecting the SARS-CoV-2 antigen
- No sample preparation step;
- Single-stage analysis;
- Compatibility with almost all transport media;
- Higher sensitivity and specificity compared to rapid tests;
- False positive results are completely excluded;
- Possibility of automation for high performance laboratories;
- The cost of one study is lower than when using rapid tests.
- E-1-AG-96
- E-2-AG-96
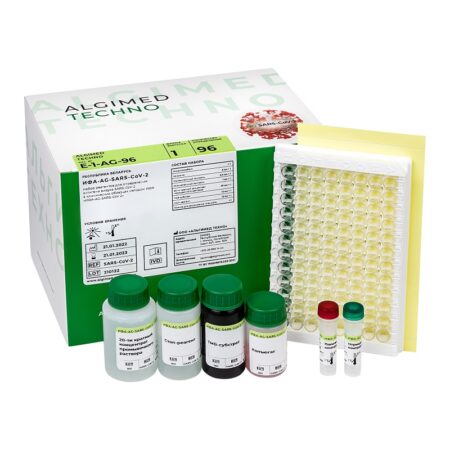
«ELISA AG SARS-CoV‑2» reagent kit (c.n. E-1-AG-96, 96 assays):
- Microtiter plate – 1 pc;
- Conjugate – 6 mL × 1 pc;
- Positive Control – 0,3 mL × 1 pc;
- Negative Control – 1 mL × 1 pc;
- Wash Buffer Concentrate (20x) – 30 mL × 1 pc;
- TMB Substrate – 12 mL × 1 pc;
- Stop Solution – 12 mL × 1 pc;
- Plate coat – 1 pc.
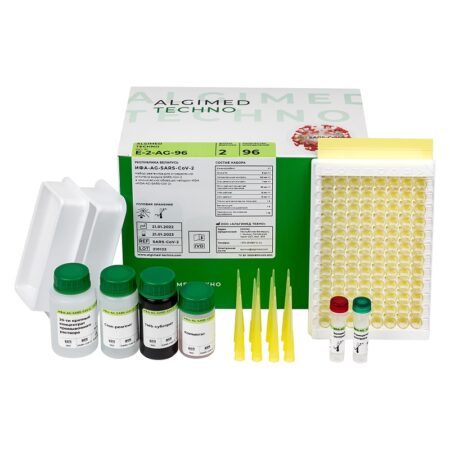
«ELISA AG SARS-CoV‑2» reagent kit (c.n. E-2-AG-96, 96 assays):
- Microtiter plate – 1 pc;
- Conjugate – 6 mL × 1 pc;
- Positive Control – 0,3 mL × 1 pc;
- Negative Control – 1 mL × 1 pc;
- Wash Buffer Concentrate (20x) – 30 mL × 1 pc;
- TMB Substrate – 12 mL × 1 pc;
- Stop Solution – 12 mL × 1 pc;
- Plate coat – 2 pcs;
- Multichannel pipettor trays – 2 pcs;
- Disposable polymer pipette tips – 16 pcs.
Based on an indirect, non-competitive sandwich ELISA. The kit uses monoclonal antibodies that are specific to two different epitopes of the SARS CoV‑2 nucleocapsid protein molecule and do not compete with each other. The test and control samples are incubated in the wells of the plate with the immobilized first monoclonal antibody in the presence of the second monoclonal antibody labeled with horseradish peroxidase. After incubation with the substrate and the application of the stop reagent, the optical density of the solution is measured. The measured optical density is directly related to the amount of antigen in the control and test samples.