IgGRBD-SARS-CoV-2 ELISA kit is now available on the EU market!
- This entry was posted:June 2, 2022
Dear colleagues!
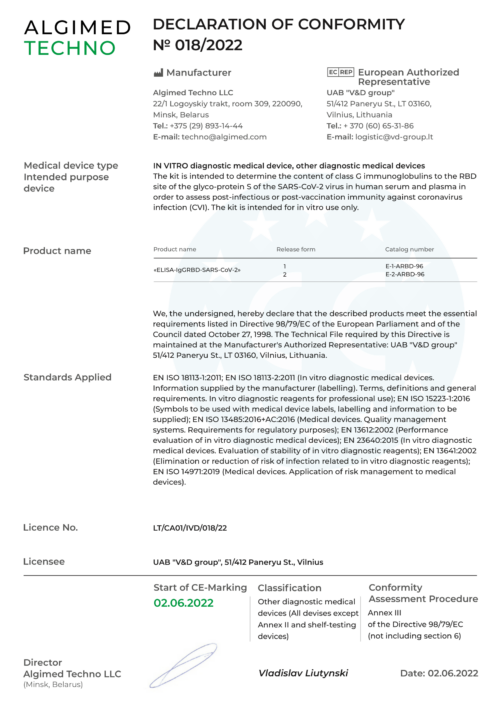
On June 2, 2022 Algimed Techno successfully passed the registration and received the certificate of conformity for IgGRBD-SARS-CoV-2 ELISA kit. The kit have been granted CE-IVD status, according to Directive 98/79/EC of the European Parliament on the treatment of medical products, thereby confirming compliance with all European Union acts and standards, its quality and safety for health.
The successfully completed registration procedure allow our company to enter the European market and to develop the export potential.
IgGRBD-SARS-CoV-2 ELISA kit is designed to determine the level of IgG to the SARS-CoV-2 glycoprotein S RBD site in human serum and plasma in order to assess post-infectious or post-vaccination immunity against coronavirus infection.
Based on the information obtained during the research using IgGRBD-SARS-CoV-2 ELISA kit, it is possible to draw conclusions about:
- Vaccination effectiveness ;
- Re-infection risk degree;
- The suitability of donor plasma for transfusion in patients with severe coronavirus infection.